One-Step TB Rapid Test,S/P
- FOB Price:Get Latest Price >
- Min.Order:100 Piece(s)
- Production Capacity:TB
- Payment Terms:T/T , PayPal , EXW
- Favorite
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


Span Biotech Ltd.
We are professional supplier of Rapid Tests,Elisa Kits,IVD,Diagnostical Reagents,Raw Material.
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank

INTENDED USE
The TB Rapid Test is a chromatographic immunoassay for direct qualitative detection of Tuberculosis antibody in human serum or plasma.
PRINCIPLE
Tuberculosis remains an important socio-economical and medical problem throughout the world. According to the Centers for Disease Control, the incidence of TB is expected to increase from 7.5 million cases per year in 1995 to 11.9 million in 2005. The case fatality rate is estimated at 55% for untreated people and 15% for the treated patients.
The commonly used diagnostic tests for the tuberculosis such as sputum examination of acid fast bascilli, culture of sputum or other fluids, the tuberculin skin test and radiological investigations do not achieve the required diagnostic sensitivity.
TB is a chromatographic immunoassay test for the detection of TB antibody in human serum or plasma. Specific antigens are pre-coated onto membrane as a capture reagent on the test region. During the test, specimen is allowed to react with the antigens firstly. The chasing buffer mixed with specific Mouse anti human antibody, which is conjugated with colloidal gold, will flow up the membrane. If TB antibody is present, a pink colored band will develop on the test region of membrane. Absence of this pink colored band in the test region suggests a negative result. To serve as a procedural control, a pink colored band in the C region will always appear regardless of the presence or absence of TB antibody.
REAGENTS AND MATERIALS PROVIDED
1. One pouched cassette with desiccant.
2. Blood diluent in a dropper bottle, stored at 2-8°C.
3. One piece of operating instruction
WARNING AND PRECAUTIONS
1.For in vitro diagnostic use only.
2.All patient samples should be treated as if capable of transmitting diseases.
3.Do not interchange reagents from different lots or use the test kit beyond expiration date.
4.Icteric, lipemic, hemolysed, heat treated and contaminated sera may give erroneous results.
STORAGE
The kits should be stored at temperature 4-30°C, the sealed pouch for the duration of the shelf life (24 months).
SAMPLE COLLECTION AND PREPARATION
Plasma
1. Have a certified phlebotomist collect whole blood into a purple, blue or green top collection tube (containing EDTA, citrate or heparin, respectively) by vein puncture.
2. Separate the plasma by centrifugation.
3. Carefully withdraw the plasma for testing, or label and store it at 2-8°C for up to two weeks. Plasma may be frozen at -20°C for up to one year.
Serum
1. Have a certified phlebotomist collect whole blood into a red top collection tube (containing no anticoagulants) by veinpuncture.
2. Allow the blood to clot.
3. Separate the serum by centrifugation.
4. Carefully withdraw the serum for testing or label and store it at 2-8°C for up o two weeks. Serum may be frozen at -20°C for up to one year.
ASSAY PROCEDURE
1. Bring all reagent to room temperature.
2. Open a pouch containing a cassette and lay the cassette on a flat surface.
3. Carefully apply 3ml of sample Into the sample well(please the picture)
4. Then add 2 drops (80ul) of buffer into the buffer well (B).
5. Read the result within or up to 15 minutes. Dispose of the cassette after 20 minutes.
INTERPRETATION OF RESULTS
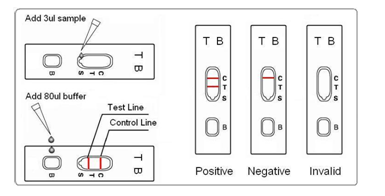
1. Negative: One pink band appears in the control region (C), no band in test region (T). This indicates that there is no detectable TB antibody in the specimen.
2. Positive: One pink band in each control & test region, indicates positive result.
3. Invalid: If no band appears in the control region(C), regardless of the presence or absence of band in the test region (T). It indicates a possible error in performing the test. The test should be repeated using a new device.
LIMITATION OF THE PROCEDURE
The test is to be used for the qualitative detection of TB antibody.
A negative result does not rule out infection by Tuberculosis because the TB antibody may not be present in sufficient quality. To confirm the disease, the result should be evaluated using in conjunction with other clinical methods.
REFERENCE:
1.Grange J.M. (1984): The human immune response in tuberculosis: its nature, biological role and diagnostic usefulness. In: Fow W, Grosset J, Styblo K. (eds)- Advances in tuberculosis research vol.21 Karger-Basel 1:78
2.Cocito C. and Valinden F. (1987): The serodiagnosis of tuberculosis and other mycobacterial disease by ELISA-Am. Rev. Respir. Dis. 135: 1137-1151
3.Toman K. (1981): Sensitivity, specificity and predictive value of diagnostic tests-Bull. Int. Union Tuberc. 56 : 18-28
4.Krambovitis E. (1987): Serodiagnosis of tuberculosis in perspective – Serdiagn. Immunoth 1: 7-19.