One-Step HBsAb Rapid Test,Cassette/Strip
- FOB Price:Get Latest Price >
- Min.Order:100 Piece(s)
- Production Capacity:HBsAb
- Payment Terms:T/T , PayPal , EXW
- Favorite
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


Span Biotech Ltd.
We are professional supplier of Rapid Tests,Elisa Kits,IVD,Diagnostical Reagents,Raw Material.
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank

FOR THE QUALITATIVE ASSESSMENT OF HBsAb IN HUMANSERUM, PLASMA OR WHOLE BLOOD
INTENDED USE
Rapid HBsAb Test is a chromatographic immunoassay for qualitative detection of the surface antibody of hepatitis B virus (Anti-HBs) in human whole blood samples. It is intended for use in medical institution as an aid for diagnosis and management of patients related to infection with hepatitis B as well for screening of blood donors or blood products
PRINCIPLE
Rapid HBsAb Test employs chromatographic lateral flow device. Colloidal gold conjugated surface antigen reactive to anti-HBs (sAg-Au) are dry-immobilized onto a nitrocellulose membrane strip. When the sample is added, it migrates by capillary diffusion through the strip rehydrating the gold conjugate. If present, anti-HBs will bind with the gold conjugated antigens forming particles. These particles will continue to migrate along the strip until the Test Zone (T) where they are captured by HBsAg immobilized there and a visible red line appears. If there is no anti-HBs in sample, no red line will appear in the Test Zone (T). The gold conjugate will continue to migrate alone until is captured in the Control Zone (C) from immobilized goat, anti-HBs antibody and aggregating in a red line, which indicates the validity of the test
STORAGE
Store the test device at 4 to 30°C. Do Not Freeze.
WARNING AND PRECAUTIONS
1. For in vitro diagnostic use only.
2. Do not use product beyond the expiration date.
3. Handle all specimens as potentially infectious.
REAGENTS AND MATERIALS PROVIDED
1. Rapid HBsAb Test
2. Instructions for use
3.Disposable transfer pipet
MATERIALS REQUIRED BUT NOT PROVIDED
1.Whole blood or plasma: Vacutainer tube, or other appropriate tube, containing heparin or EDTA as an anticoagulant
2.Serum: Vacutainer tube, or other appropriate tube, without anticoagulant
3. Timer or clock
SAMPLE COLLECTION AND PREPARATION
1.The serum, plasma or whole blood specimen should be collected under standard laboratory conditions.
2.Heat inactivation of specimens, which may cause hemolysis and protein denaturation, should be avoided.
3.Patient samples performed best when tested immediately after collection. If specimans are to be stored, the red blood cells should be removed to avoid hemolysis. If the sample cannot be tested within 24 hours, serum or plasma should be frozen until the test can be performed. Allow sample to reach room temperature before proceeding.
4.Sodium azide can be added as a preservative up to 0.1% without effecting the test results.
ASSAY PROCEDURE
1. Bring all materials and specimens to room temperature.
2. Remove the test cassette from the sealed foil pouch.
3.Label the test cassette with specimen identity by writing the ID on the top label of the strip.
4. Place the test cassette on a flat horizontal surface.
5. Using the transfer pipet to draw up the sample.
6. Hold the transfer pipet in a vertical position over the sample pad and dispense 2-3 drops (80-120 ml) of sample onto the sample pad.
7. Read the result at 20 minutes after adding the sample.
Note: Some positive samples may show positive results before 20 minutes. Results after 30 minutes may not be accurate.
QUALITY CONTROL
An internal procedural control is included in the test. A red-purple line will appears in the control region (C), which confirms sufficient specimen volume and correct operation for the test.
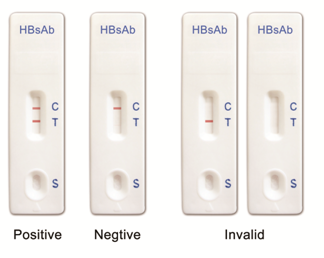
INTERPRETATION OF RESULTS
Positive:
Two colored bands appear within 20 minutes. One colored band appears in the Control Zone (C) and another colored band appears in the Test Zone (T). The test result is positive and valid. No matter how faint the colored band appears in the Test Zone (T), the test result should be considered as positive result.
Negative:
One colored bands appears in the Control Zone (C) within 20 minutes. No colored band appears in the Test Zone (T). The test result is negative and valid.
Invalid result:
No colored band appears in the Control Zone (C) within 20 minutes. The test result is invalid. Repeat the test with a new test device.
PERFORMANCE CHARACTERISTICS
In a clinical evaluations of the performance of the Rapid HBsAb Test:
1.Interferring factors
Presence of 0.4g/dL hemoglobin, 20mg/dL bilirubin, 1.95nmol/L triglyceride, or 6.10nmol/L cholesterol don’t affect the detection of anti-HBs.
2.Comparison with the ELISA method
The results of the HBsAb test strip in total of 1249 samples (535 ELISA confirmed positive specimens and 714 ELISA confirmed negative specimens) is summarized.
Positive agreement(%) Negative agreement(%) Accuracy (%) |
97.6 (522/535) 98.2 (701/714) 97.9 (1223/1249) |
Packaging Details:
Pouch+Box+Carton packaging
(1) With our company’s Logo
(2) With the natural package
(3) With OEM package
(4) ODM