One-Step H. pylori Ab Rapid Test,Cassette/Strip
- FOB Price:Get Latest Price >
- Min.Order:100 Piece(s)
- Production Capacity:H.P
- Payment Terms:T/T , PayPal , EXW
- Favorite
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


Span Biotech Ltd.
We are professional supplier of Rapid Tests,Elisa Kits,IVD,Diagnostical Reagents,Raw Material.
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank

INTRODUCTION
One Step H. Pylori Ab wb /serum/plasma test is a rapid test for the qualitative detection of antibodies specific to Helicobacter pylori in human serum/plasma and whole blood. In this test kit, the H. pylori antigen – colloid gold conjugate and specimen moves along the membrane chromatographically to the test region and forms a visible line as the antigenantibodyantigen gold particle complex forms with high degree of sensitivity and specificity. This one step test only takes about 1015 minutes. Test results are read visually without any instrumentation.
SPECIMEN COLLECTION & PREPARATION
Both whole blood (with or without anticoagulant), serum and plasma specimens can be used with this assay. Simply follow the standard clinical produres to collect whole blood, serum or plasma specimens. If the specimen cannot be tested on the day of collection, store the serum/plasma specimen in a temperature of 28 ºC for up to 72 hours. Stir and bring the specimens to room temperature before testing. Do not freeze whole blood specimens. Do not freeze and thaw the specimen repeatedly. Attention: Specimens and all materials coming in contact with them should be handling and disposed of as if capable of transmitting infection. Avoid contact with skin by wearing gloves and proper laboratory attire.
REAGENTS AND MATERIALS SUPPLIED
1. Test kit
2. Specimen diluents in dropper bottle
3. Plastic dropper to dispense sample
TEST PROCEDURE
1. When you are ready to begin testing, open the sealed pouch by tearing along the notch. Remove the test device from the pouch and use it as soon as possible.
2. Using the plastic dropper provided, draw the sample into the pipette. Dispense one drop of sample (~25μL) onto the sample well of the cassette.
3. Add 3 drops of diluents (150μL) provided onto the sample well. Use the dropper bottle provided, not the sample pipette.
4. Wait for 10 minutes and read results. It is important that the background is clear before the result is read. Do not read results after 15 minutes.
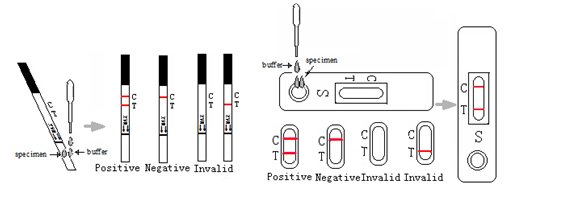
INTERPRETATION OF RESULTS
Negative: Only one colored band appears on the control (C) region. No apparent band on the test (T) region.
Positive: In addition to a pink colored control (C) band, a distinct pink colored band will appear in the test (T) region.
Invalid: A total absence of color in both regions or no colored line appears in the control (C) region is an indication of procedure error and/or test reagent deterioration. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
LIMITATIONS
1.As with all diagnostic tests, all results must be considered with other clinical information available to the physician. A definite clinical diagnosis should only be made by the physician after all clinical and laboratory findings have been evaluated.
2. The test should be used for the detection of antibodies to H. pylori in whole blood/serum/plasma specimen.
3. This kit will only indicate the presence of H. pylori antibody in the specimen and should not be used as the sole criteria for the diagnosis of H. pylori infection.
4. If the test result is negative and clinical symptomspersist, additional testing using other clinical methods is recommended. A negative result does not at any time preclude the possibility of H. pylori infection.
STORAGE AND STABILITY
The test kit can be stored at temperatures between 2 to 30ºC in the sealed pouch to the date of expiration.
The test kit should be kept away from direct sunlight, moisture and heat.
PRECAUTION
1. For in vitro diagnostic use only.
2. Do not use test kit beyond expiry date.
3. The test device should not be reused.
4. Do not compare results from a different device.
5. Avoid crosscontamination of specimens by using a new specimen collection container and specimen pipette for each sample.
6. Specimens may be infectious. Proper handling and disposal methods should be established.