One-Step HIV Ab/Ag Combo Rapid Test,Cassette/Strip
- FOB Price:Get Latest Price >
- Min.Order:100 Piece(s)
- Production Capacity:HIV
- Payment Terms:T/T , PayPal , EXW
- Favorite
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


Span Biotech Ltd.
We are professional supplier of Rapid Tests,Elisa Kits,IVD,Diagnostical Reagents,Raw Material.
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank

INTENDED USE
The HIV (1+2) Ag/Ab combo rapid test kit is a rapid, qualitative test for the detection of HIV p24 antigen and antibodies to all isotypes (IgG, IgM and IgA) specific to HIV-1 including subtype-O and/or HIV-2 simultaneously in human serum, plasma. The antigens are prepared with proteins to have HIV-1, gp41, gp120 and gp36. For HIV 2, p24 ab & gp36. The HIV (1+2) Ag/Ab Combo Test kit (3lines) is intended for professional use, only for an initial screening test and reactive samples should be confirmed by a supplemental assay such as ELISA or Western Blot test.
PRECAUTION
1.For in vitro diagnostic uses only.
2.Clean up spills thoroughly using an appropriate disinfectant.
3.Decontaminate and dispose of all specimens, reaction kits and potentially contaminated materials, as if they were infectious waste, in a biohazard container.
4.Do not mix and interchange different specimen.
5.Anticoagulants such as heparin, EDTA and sodium citrate do not affect the test result.
6.Lcteric, lipemic, hemolysed, heat treated and contaminated specimens may give erroneous results.
7.Do not use test kit beyond expiration date.
SUMMARY AND EXPLANATION OF THE TEST
HIV (Human Immunodeficiency Virus) is recognized as the etiologic agent of Acquired immune Deficiency Syndrome (AIDS). The virus is transmitted by sexual contact, exposure to infected blood, certain body fluids or tissues, and from mother to fetus or child during the perinatal period. HIV-1 has been isolated from patients with high potential risk of developing AIDS.
HIV-1 appears to be the most common one while HIV-2 is mostly found in patient samples from West African region. Its course is marked by increasing levels of viral replication and the emergence of more virulent viral strains. This process causes the destruction of the immune system. HIV infection is staged by CD4 cell counts and clinical symptoms. Not all people progress through all “stages” and the time frames may also very greatly from person to person.
PRINCIPLE
The 4th generation HIV(1+2) Ag/Ab Combo Test kit (3lines) contains a membrane strip, which is pre-coated with mouse monoclonal Anti-HIV p24 antibody on test line “2” region with gp36 and test line “1” region with recombinant HIV-1/HIV-2 antigens respectively. The mixture of mouse monoclonal anti-HIV p24 antibody and recombinant HIV-1/HIV-2 antigen-colloid gold conjugate form a visible lines (1&2) as the antigen-antibody-antigen or antibody-antigen-antibody gold particle complex with high degree of sensitivity and specificity.
The test lines and control line in the result window have been clearly labeled. For line “2” is for HIV p24 antigen and/or antibodies to HIV-2 and line “2” is for antibodies to HIV-1/HIV-2, and “C” for Control line. All the test lines and control line in the result window are not visible before applying any sample. The control line is used for procedural control and should always appear if the test procedure is performed correctly.
MATERIALS SUPPLIED
1.Forty pouched cassettes with desiccant and dropper.
2.Blood diluent in a dropper bottle, stored at 2-8°C.
3.One piece of operating instruction.
MATERIALS NOT PROVIDED BUT REQUIRED
1.Specimen collection container, either plastic or class.
2.Timer.
3.Disposable gloves.
SPECIMENS COLLECTION AND STORAGE
Plasma
1.Have a certified phlebotomist collect whole blood into a purple, blue or green top collection tube (containing EDTA, citrate or heparin, respectively) by veinpuncture.
2.Separate the plasma by centrifugation.
3.Carefully withdraw the plasma for testing, or label and store it at 2-8°C for up to two weeks. Plasma may be frozen at -20°C for up to one year.
Serum
1.Have a certified phlebotomist collect whole blood into a red top collection tube (containing no anticoagulants) by veinpuncture.
2.Allow the blood to clot.
3.Separate the serum by centrifugation.
4.Carefully withdraw the serum for testing or label and store it at 2-8°C for up to two weeks. Serum may be frozen at -20°C for up to one year.
ASSAY PROCEDURE
Add 2 drop (1 drop = 35µl) of sample (serum/plasma) into the sample well. Observe the result in 5-15 minutes.
QUALITY CONTROL
An internal procedural control is included in the test. Red line will appears in the control region (C).
INTERPRETATION OF RESULTS
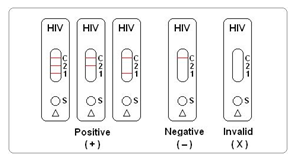
Negative: No apparent band in the test region (1 and 2), Only one red band appears in the control region (C). This indicates that no HIV p24 antigen and/or antibodies to HIV-1/HIV-2 have been detected.
Positive:
1) The presence of two lines as control line “C” and test line “2” within the result window indicates a positive result for HIV p24 antigen . The presence of HIV antigen suggests that the infection is at an early stage.
2) The presence of two lines as control line “C” and test line “1” within the result window indicates a positive result for antibodies to HIV-1/HIV-2.
3) The presence of three lines as control line “C”, test line “2” and test line “1” within the result window indicates a positive result for HIV p24 antigen and/or antibodies to HIV-1/HIV-2. The presence of HIV antigen suggests that the infection is at an early stage.
Invalid: If no band appears in the control region(C), regardless of the presence or absence of line in the test region (T1 & T2). It indicates a possible error in performing the test. The test should be repeated using a new device.
Note: Except the red line, no other color line means a positive result. Do not read the results after 30 minutes. Dispose off the used cassette as infectious material.
PERFORMANCE CHARACTERISTICS
Analytical Sensitivity
The detection limit of the HIV(1+2) Ag/Ab Combo Test kit (3lines) is 2IU/ml, which is evaluated by testing the HIV-1 p24 antigen panel from the National institute for Biological Standard and Control.
STORAGE AND STABILITY
Store the 4th generation HIV(1+2) Ag/Ab Combo Test Kit (3lines) at temperature ranges 2-30°C in the sealed pouch for the duration of the shelf life period (24months). Refer to the expiration date for stability. Do not freeze.
LIMITATION
1.The test is to be used for the qualitative detection of antibodies to HIV.
2.A negative result does not rule out infection by HIV because the antibodies to HIV may be absent or may not be present in sufficient quality to be detected at early stage of infection.
3. A positive result, even a weak positive, must be verified with a confirmation test.
4.As with all diagnostic tests, the result must be correlated with clinical findings. If the test result is negative and suspicion still exists, additional follow-up testing using other clinical methods is recommended.