One-Step HCV Ab 4th Gen. Rapid Test,Cassette/Strip
- FOB Price:Get Latest Price >
- Min.Order:100 Piece(s)
- Production Capacity:HCV
- Payment Terms:T/T , PayPal , EXW
- Favorite
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


Span Biotech Ltd.
We are professional supplier of Rapid Tests,Elisa Kits,IVD,Diagnostical Reagents,Raw Material.
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank

INTENDED USE
The HCV Gold Rapid Screen Test (RST) is a Chromatographic immunoassay (CIA) for direct qualitative detection of antibodies to Hepatitis C type virus (HCV) in human serum/ plasma and whole blood.
PRINCIPLE
HCV RST is a chromatographic immunoassay (CIA) for the detection of antibodies to HCV in human serum/plasma and whole blood. HCV recombinant antigens are precoated onto membrane as a capture reagent on the test region. During the test, specimen is allowed to react with the colloidal gold particles, which have been labeled with HCV recombinant antigens. If antibodies to HCV are present, a pink colored band will develop on the membrane in proportion to the amount of HCV antibodies present in the specimen. Absence of this pink colored band in the test region suggests a negative result. To serve as a procedural control, a pink colored band in the control region will always appear regardless the presence of antibodies to HCV.
REAGENTS AND MATERIALS PROVIDED
1. One sealed pouched cassette with desiccant &disposable pipettet.
2.Blood diluent in a dropper bottle. Store at 4-30°C.
3.One piece of operating instruction with 40 test pouches.
WARNING AND PRECAUTIONS
1.For in vitro diagnostic uses only.
2.All patient samples should be treated as if capable of transmitting diseases.
3.Do not interchange reagents from different lots. Do not use test kit beyond expiration date.
4.Icteric, lipemic, hemolysed, heat treated and contaminated sera may cause erroneous results.
STORAGE
The kits (the sealed pouch) should be stored at room temperature ( 4-30°C), for the duration of the shelf life (24 months).
SAMPLE COLLECTION AND PREPARATION
Whole Blood ( Finger stick Specimens)
1.Clean the area to be lanced with an alcohol swab.
2.Squeeze the end of the fingertip and pierce it with a sterile lancet.
3.Wipe away the first drop of blood with sterile gauze or cotton.
4.Use micropipette to obtain about 100ul fresh blood and dispense into the sample well.
Plasma
1.Have a certified phlebotomist collect whole blood into a purple, blue or green top collection tube (containing EDTA, citrate or heparin, respectively) by veinpuncture.
2.Separate the plasma by centrifugation.
3.Carefully withdraw the plasma for testing, or label and store it at 2-8°C for up to two weeks. Plasma may be frozen at -20°C for up to one year.
Serum
1.Have a certified phlebotomist collect whole blood into a red top collection tube (containing no anticoagulants) by veinpuncture.
2.Allow the blood to clot.
3.Separate the serum by centrifugation.
4.Carefully withdraw the serum for testing or label and store it at 2-8°C for up to two weeks. Serum may be frozen at -20°C for up to one year.
ASSAY PROCEDURE
Serum or Plasma Sample
Collect 100-150ml or 2-3 drops of serum or plasma in a sample well,Observe the result in 5-30 minutes.
Whole Blood Sample
Add 1 drop (1 drop = 35µl) of sample (whole blood) into the sample well. After all samples completely absorbed, add 1 drop of diluent. Observe the result in 5-30 minutes.
INTERPRETATION OF RESULTS
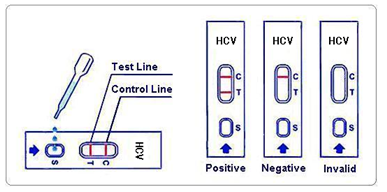
1.Negative: No apparent band in the test region (T), a pink-colored band appears in the control region (C). This indicates that no HCV antibody has been detected.
2.Positive: In addition to a pink-colored band in the control region (C), a pink-colored band will appear in the test region (T). This indicates that the specimen contains HCV antibodies.
3.Invalid: If no band appears in the control region (C), regardless of the presence or absence of line in the test region (T). It indicates a possible error in performing the test. The test should be repeated using a new device.
PERFORMANCE CHARACTERISTICS
10100 HCV diagnostic samples were tested with the HCV Rapid Screen Test. The results are summarized in the table below.
Diagnostic Sample type | Number of sample tested | HCV Rapid Screen Test | |
negative | Positive | ||
Positive | 523 | 8(8) | 515(A) |
Negative | 9577 | 9542(D) | 35(C ) |
Sensitivity=98.5; Specificity=99.6%; Total accuracy=99.6%
Precision
Intra-assay
In the study, 30 replicate assays were performed with one positive and one negative specimen. Correct negative and positive results were registered in 100% of the assays.
Inter-assay
The study involved the same specimens containing one positive and one negative. The samples were analyzed in 30 independent assays with HCV Rapid Screen Test. originating from three different lots at different times during two months. Again, expected negative and positive results were registered in 100% of the assays.
STORAGE AND STABILITY
Store HCV Rapid Screen Test at temperature ranges 4-30 °C in the sealed pouch. Refer to the expiration date for stability. Do not freeze.
LIMITATION OF THE PROCEDURE
1.The test is to be used for the qualitative detection of antibodies to HCV.
2. A negative result does not rule out infection by HCV because the antibodies to HCV may be absent or may not be present in sufficient quantity to be detected at early stage of infection.
PRODUCT DISCLAIMER
This product has been manufactured under strict GMP regulation so to ensure the diagnostic accuracy of the test. It is out of control of the manufacture when the test is performed in diverse environment and by diverse group of individuals which may affect the result to a certain degree.
NOTE
The manufacture, the Distributor or its associates will not be liable for any losses, claims, liability, costs or damages, whether direct or indirect or consequential arising out of or related to an incorrect diagnosis, whether a positive or negative by use of this product.