One-Step Influenza A/B Rapid Test,Cassette/Strip
- FOB Price:Get Latest Price >
- Min.Order:100 Piece(s)
- Production Capacity:Influenza
- Payment Terms:T/T , PayPal , EXW
- Favorite
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


Span Biotech Ltd.
We are professional supplier of Rapid Tests,Elisa Kits,IVD,Diagnostical Reagents,Raw Material.
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank

Intended Use
The Influenza A +B Test is a rapid chromatographic immunoassay for the qualitative detection of influenza type A and B nucleoprotein antigens in nasopharyngeal swab and nasal aspirate specimens. This kit is intended to be used as an aid in the diagnosis of influenza A and influenza B virus in nasal swab specimens.
Principle
The Influenza A +B Test is a qualitative membrane strip based immunoassay for the detection of influenza type A and B nucleoprotein antigens in nasopharyngeal swab and nasal aspirate specimens. In this test procedure, influenza type A antibody is immobilized in the A line, influenza type B antibody is immobilized in the B line. After a specimen is placed in the specimen well, it reacts with influenza type A antibody and/or B antibody coated particles that have been applied to the specimen pad. This mixture migrates chromatographically along the length of the test strip and interacts with the immobilized antibody. If the specimen contains influenza type A, a colored line will appear in the A line region indicating a influenza type A positive result. If the specimen contains influenza type B, a colored line will appear in the B line region indicating a influenza type B positive result. Absence of any T lines (A and B) suggests a negative result. To serve as a procedural control, a colored line will always appear at the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
Storage and Stability
1.Store as packaged in the sealed pouch at room temperature or refrigerated (4-30℃ or 40-86℉). The test device is stable through the expiration date printed on the sealed pouch.
2.The test must remain in the sealed pouch until use.
Additional Special Equipment
Materials Provided
Test devices Extraction Tube
Sterilized Swab Buffer
Package Insert
Materials Required But Not Provided
Timer
Precautions
1.For professional in vitro diagnostic use only. Do not use after expiration date.
2.Do not eat, drink or smoke in the area where the specimens and kits are handled.
3.Handle all specimens as if they contain infectious agents.
4.Observe established precautions against microbiological hazards throughout all procedures and follow the standard procedures for proper disposal of specimens.
5.Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are assayed.
6.Follow standard biosafety guidelines for handling and disposal of potential infective material.
7.Humidity and temperature can adversely affect results.
Specimen Collection
It is applicable to the diagnosis of the influenza virus A and B from the specimens of nasal swabs, throat swabs or nasal aspirates. Use freshly collected specimens for optimal test performance. Inadequate specimen collection or improper specimen handling may yield a false-negative result.
1.Nasal Swabbing:Completely insert the sterilized swab supplied in this kit into the nasal basin, and swab several times to collect the epidermal cells of the mucus. It is recommended to collect specimen from nasal basin for more accurate results.
2.Throat Swabbing:Deeply insert the sterilized swab into the throat and swab several times to collect the epidermal cells of the mucus. Caution has to be paid to avoid the swab to be contaminated with saliva.
3.Specimen collected may be stored for 1 days at 2-8℃ if not tested within 1 hours. For long term storage, specimens should be kept below -20℃.
Specimen Preparation
1.Add 0.5 ml of the specimen extraction buffer into the extraction tube, and put it on the tube stand.
2.Insert the swab into the extraction tube which contains 0.5 ml of the extraction buffer. Rotate the swab inside the tube using a circular motion to roll the side of the extraction tube so that the solution is expressed and reabsorbed from the swab. The extracted solution will be used as test specimen.
Test Procedure
Allow the test, specimen and/or controls to reach room temperature 15-30℃ (59-86℉) prior to testing.
1.Bring the pouch to room temperature before opening it. Remove the test device from the sealed pouch and use it as soon as possible.
2.Place the test device on a clean and level surface.
3.Insert a nozzle with filter into the specimen extraction tube tightly.
4.Reverse the specimen extraction tube, Holding the specimen extraction tube upright, transfer 3 drops (approximately 100μl) to the specimen well(S) of the test device, then start the timer. See illustration below.
5.Wait for the colored line(s) to appear. Read results at 15 minutes. Do not interpret the result after 20 minut .
Notes:
Applying sufficient amount of specimen is essential for a valid test result. If migration (the wetting of membrane) is not observed in the test window after one minute, add one more drop of specimen to the specimen well.
Interpretation of Results
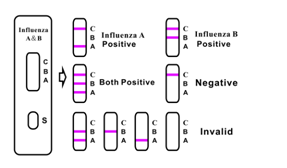
Positive: Control line and at least one test line appear on the membrane. The appearance of A test line indicates the influenza type A positive. The appearance of B test line indicates the influenza type B positive. And if both A and B line appear, it indicates that the presence of both influenza type A and B positive. The lower the antigen concentration is, the weaker the result line is.
Negative: One colored line appears in the control region(C).No apparent colored line appear in the test line region.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Limitations
1.The Influenza A +B Test is for in vitro diagnostic use only. The test should be used for the detection of influenza type A and B nucleoprotein antigens in nasopharyngeal swab and nasal aspirate specimens only. Neither the quantitative value nor the rate of increase in influenza type A or B can be determined by this qualitative test.
2.The Influenza A +B Test will only indicate the presence of influenza type A and B in the specimen and should not be used as the sole criteria for the diagnosis of influenza type A and B.
3.As with all diagnostic tests, all results must be interpreted together with other clinical information available to the physician.
4.If the test result is negative and clinical symptoms persist, additional testing using other clinical methods is recommended. A negative result does not at any time preclude the possibility of influenza type A or B infection.